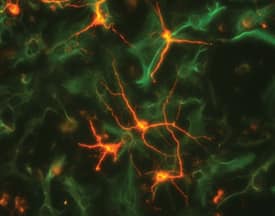
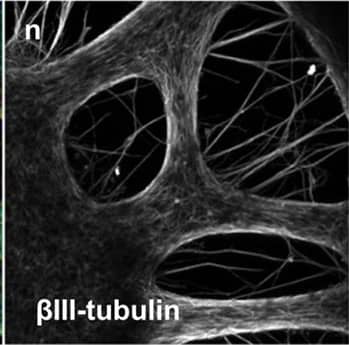
beta‑III Tubulin and Nestin in Rat Cortical Stem Cells.
beta-III Tubulin and Nestin were detected in rat cortical stem cells (Catalog #
NSC001) using 5 µg/mL neuron-specific Mouse Anti-Neuron-specific beta-III Tubulin Monoclonal Antibody (Catalog # MAB1195) and 10 µg/mL Rat Nestin Antigen Affinity-purified Polyclonal Antibody (
AF2736). Cells were incubated with primary antibodies for 3 hours at room temperature. Cells were stained for beta-III Tubulin using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red;
NL007) and for Nestin using the NorthernLights 493-conjugated Anti-Goat IgG Secondary Antibody (green;
NL003). Tissue was counterstained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
beta‑III Tubulin in Human Brain.
beta-III Tubulin was detected in immersion fixed paraffin-embedded sections of human brain (cerebellum) using Mouse Anti-Neuron-specific beta-III Tubulin Monoclonal Antibody (Catalog # MAB1195) at 8 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Mouse IgG VisUCyte™ HRP Polymer Antibody (
VC001). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to Purkinje neurons. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
Detection of Rat beta-III Tubulin by Simple WesternTM.
Simple Western lane view shows lysates of rat cortical neurons, loaded at 0.2 mg/mL. A specific band was detected for beta‑III Tubulin at approximately 56 kDa (as indicated) using 10 µg/mL of Mouse Anti-Neuron-specific beta‑III Tubulin Monoclonal Antibody (Catalog # MAB1195). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Detection of beta-III Tubulin in Human Brain Cerebellum.
beta-III Tubulin was detected in immersion fixed paraffin-embedded sections of Human Brain Cerebellum using Mouse Anti-Neuron-specific beta-III Tubulin Monoclonal Antibody (Catalog # MAB1195) at 1 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Mouse IgG VisUCyte™ HRP Polymer Antibody (Catalog #
VC001). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using VisUCyte Antigen Retrieval Reagent-Basic (Catalog #
VCTS021). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to Purkinje neurons. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
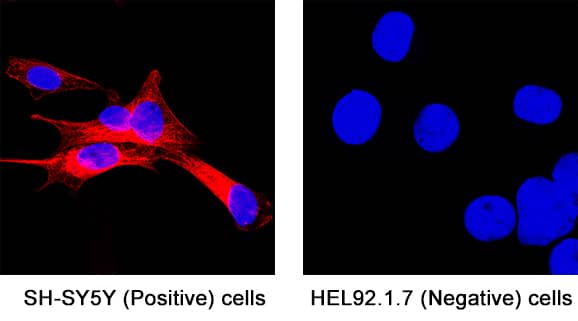
Detection of beta-III Tubulin in SH-SY5Y cells (Positive) & HEL 92.1.7 cells (Negative).
beta-III Tubulin was detected in immersion fixed SH-SY5Y human neuroblastoma cells (Positive) & absent in HEL 92.1.7 human erythroleukemic cells (Negative) using Mouse Anti-Neuron-specific beta-III Tubulin Monoclonal Antibody (Catalog # MAB1195) at 0.5 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red; Catalog #
NL007) and counterstained with DAPI (blue). Specific staining was localized to cell cytoplasm. View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
Detection of beta-III Tubulin in HepG2 cells by Flow Cytometry.
HepG2 cells were stained with Mouse Anti-Neuron-specific beta-III Tubulin Monoclonal Antibody (Catalog # MAB1195, filled histogram) or isotype control antibody (Catalog #
MAB003, open histogram), followed by Allophycocyanin-conjugated Anti-Mouse IgG Secondary Antibody (Catalog #
F0101B). To facilitate intracellular staining, cells were fixed and permeabilized with FlowX FoxP3 Fixation & Permeabilization Buffer Kit (Catalog #
FC012). View our protocol for Staining Intracellular Molecules.
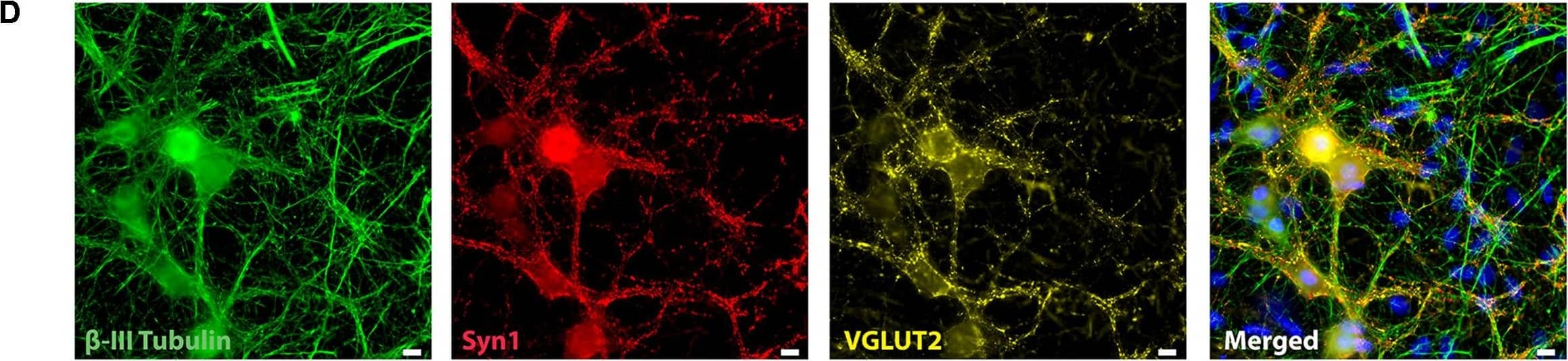
Detection of Mouse beta-III Tubulin by Immunocytochemistry/Immunofluorescence
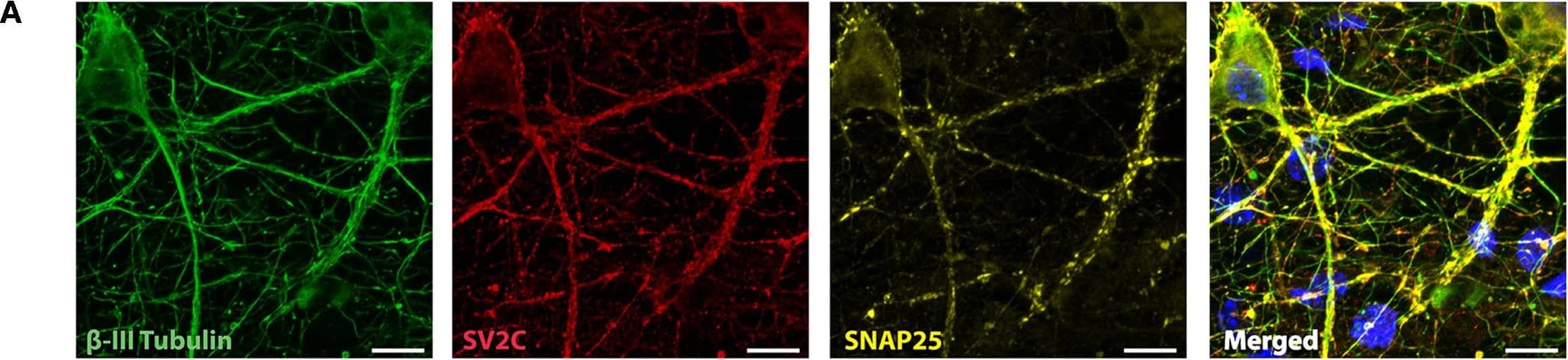
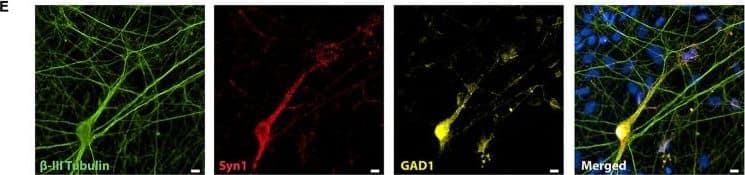
Representative immunocytochemistry demonstrating the expression of neuronal, synaptic, glutamatergic, GABAergic, and glial markers in cultures 21 days after plating. Neuronal cultures were immunostained against beta-III Tubulin (A–F); SNAP-25 (A); SV2 isoform A–C (A,B); polysialo gangliosides GD1a and GT1b/2b (C) Syn1 (D,E); glutamatergic neurons (VGLUT2, D); GABAergic neurons (GAD1, E) and glial cells (GFAP, F). Shown also are DAPI nuclear staining and the merged images. Scale bar is 10 μm (A–E), respectively, 25 μm for the bottom panel (F). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28280466), licensed under a CC-BY license. Not internally tested by R&D Systems.
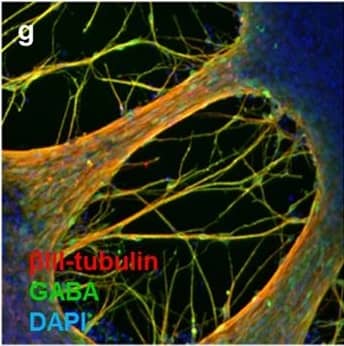
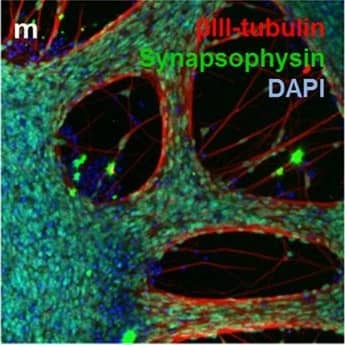
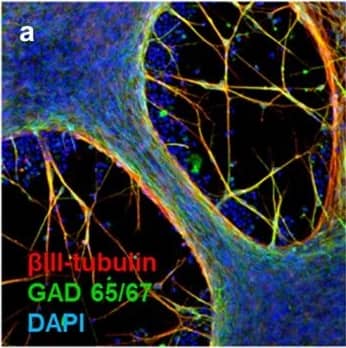
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Neuronal markers expressed by iPSC-NSCs after differentiation and maturation on PEG hydrogels.(a) betaIII-tubulin (red), GAD 65/67 (green), and DAPI (nuclei, blue). Single channel grayscale images from (a) are shown for (b) betaIII-tubulin and (c) GAD 65/67. (d) VGLUT2 (red), MAP2 (green), and DAPI (nuclei, blue). Single channel grayscale images from (d) are shown for (e) VGLUT2 and (f) MAP2. (g) betaIII-tubulin (red), GABA (green), and DAPI (nuclei, blue). Single channel grayscale images from (g) are shown for (h) betaIII-tubulin and (i) GABA. (j) MNX1/HB9 (green) and DAPI (nuclei, blue). Single channel grayscale images from (j) are shown for (k) MNX1/HB9 and (l) DAPI. (m) betaIII-tubulin (red), Synapsophysin (green), and DAPI (nuclei, blue). Single channel grayscale images from (m) are shown for (n) betaIII-tubulin and (o) Synapsophysin. (p) Synapsin-1 (green) and DAPI (nuclei, blue). Single channel grayscale images from (p) are shown for (q) Synapsin-1 and (r) DAPI. Scale Bars: 200 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26411797), licensed under a CC-BY license. Not internally tested by R&D Systems.
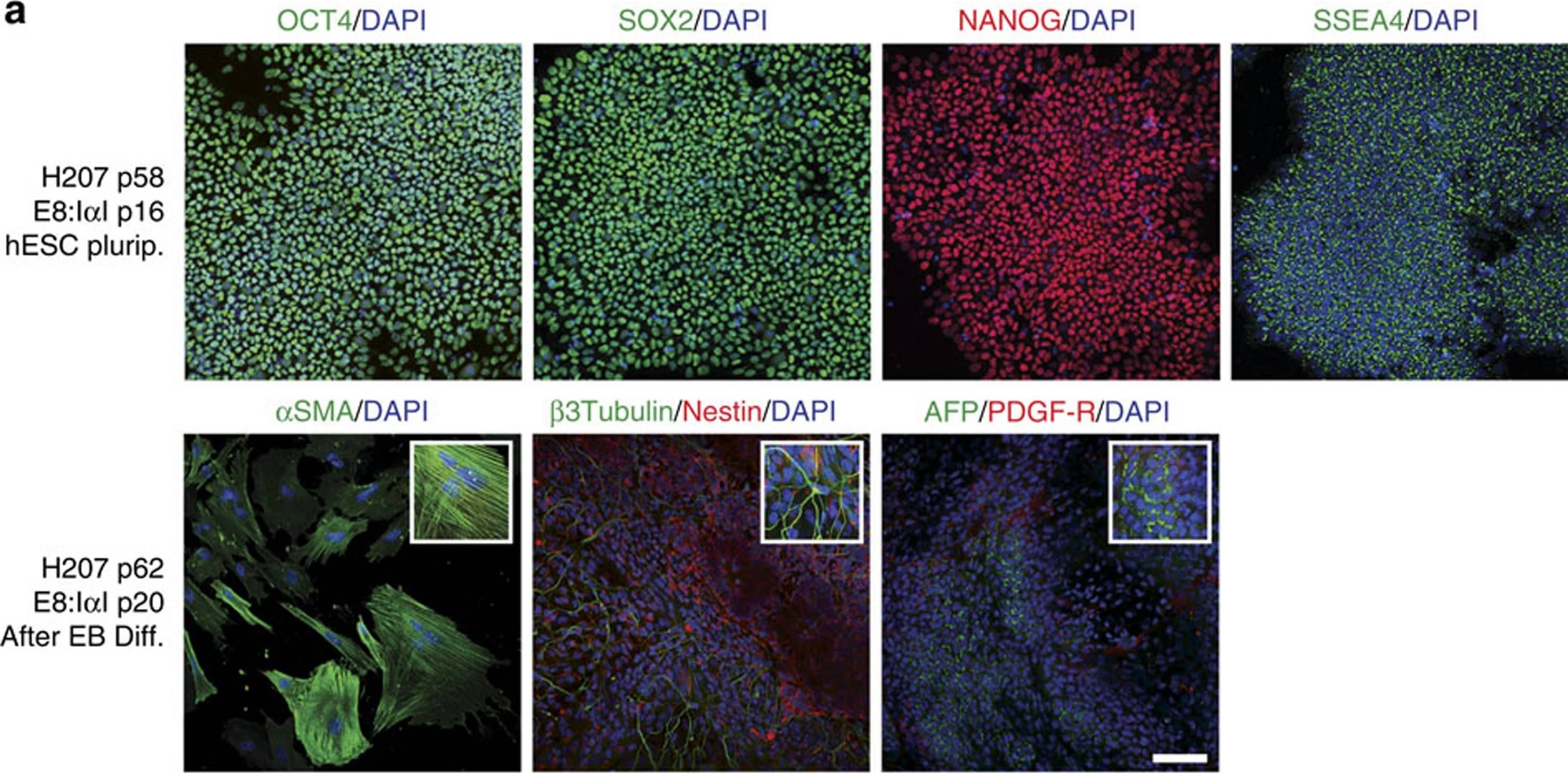
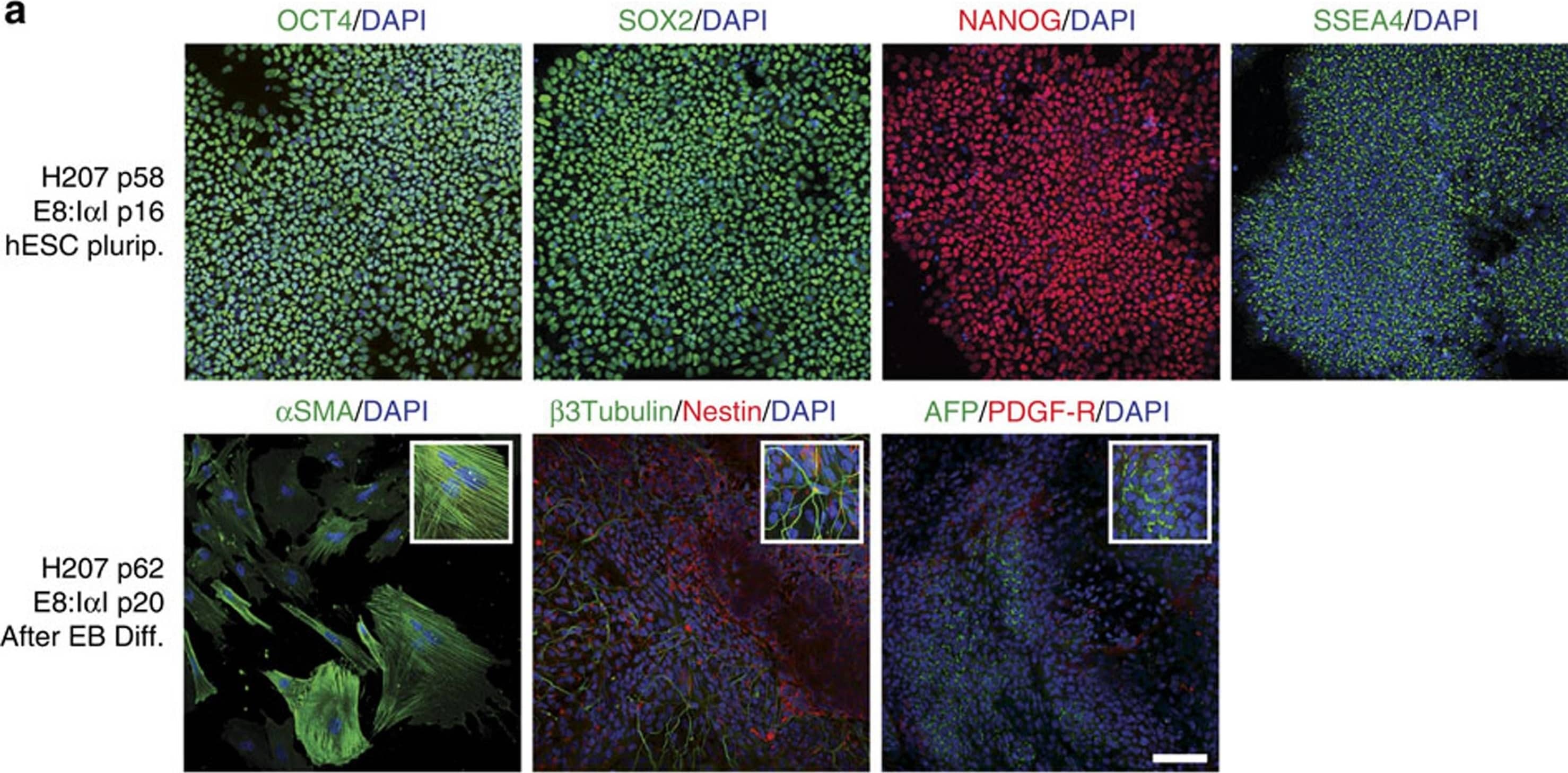
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
I alphaI maintains pluripotency and differentiation capacity in long-term culture.Immunofluorescence staining of H207 hES cell line for (a) stem cell markers Oct4 (green), Sox2 (green), Nanog (red) and SSEA-4 (green) after 16 passages in E8:I alphaI, and for the three germ layers after 4 weeks of differentiation through embryoid body (EB) formation: mesoderm with alpha smooth muscle actin (SMA, green), ectoderm with Nestin (red) and beta-III-tubulin (green) and endoderm with alpha-fetoprotein (AFP, green) and PDGF-receptor (red), and (b) after endoderm directed differentiation for endoderm markers Sox7 (red), Sox17 (green) and HNF3 beta (green) and stem cell markers Oct4 (green) and Nanog (red), as well as negative control with secondary antibodies. All samples were co-stained with DAPI (Blue) for nuclei detection. Scale bars show 100 μm, white boxes show close-up images. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/ncomms12170), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Neuronal markers expressed by iPSC-NSCs after differentiation and maturation on PEG hydrogels.(a) betaIII-tubulin (red), GAD 65/67 (green), and DAPI (nuclei, blue). Single channel grayscale images from (a) are shown for (b) betaIII-tubulin and (c) GAD 65/67. (d) VGLUT2 (red), MAP2 (green), and DAPI (nuclei, blue). Single channel grayscale images from (d) are shown for (e) VGLUT2 and (f) MAP2. (g) betaIII-tubulin (red), GABA (green), and DAPI (nuclei, blue). Single channel grayscale images from (g) are shown for (h) betaIII-tubulin and (i) GABA. (j) MNX1/HB9 (green) and DAPI (nuclei, blue). Single channel grayscale images from (j) are shown for (k) MNX1/HB9 and (l) DAPI. (m) betaIII-tubulin (red), Synapsophysin (green), and DAPI (nuclei, blue). Single channel grayscale images from (m) are shown for (n) betaIII-tubulin and (o) Synapsophysin. (p) Synapsin-1 (green) and DAPI (nuclei, blue). Single channel grayscale images from (p) are shown for (q) Synapsin-1 and (r) DAPI. Scale Bars: 200 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26411797), licensed under a CC-BY license. Not internally tested by R&D Systems.
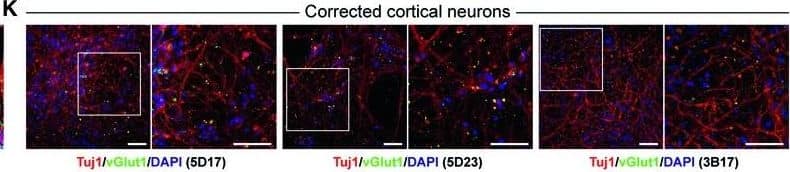
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
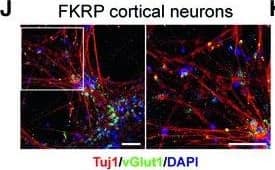
Characterization of NSCs and cortical neurons derived from FKRP‐ and CRISPR/Cas9 corrected‐iPSCsA, BRepresentative images of NSCs derived from FKRP‐ and corrected‐iPSC lines expressing SOX1, SOX2, and nestin.C, DQuantification of percentage of SOX1+ (C) and SOX2+ (D) cells in culture. The efficiency of neural induction is more than 99% in FKRP‐ and corrected‐iPSC (5D17, 5D23, and 3B17) lines. Data are mean ± s.d. n = 4 technical replicates.E, FFKRP‐ and corrected‐NSC lines can be further differentiated to cortical neural progenitor cells, expressing PAX6, OTX2, and vimentin.G–IQuantification of percentage of PAX6+ (G) and OTX2+ (H) cells in culture. About 91‐98% of cells derived from FKRP, 5D17, 5D23, and 3B17 NSC lines express PAX6 (G). About 93‐96% of cells derived from FKRP, 5D17, 5D23, and 3B17 NSC lines express OTX2 (H). Of the OTX2+ population, about 60‐67% cells are also Ki67+ cycling progenitors (I). Data are mean ± s.d. n = 4 technical replicates.J, KGlutamatergic projection neurons derived from FKRP and corrected (5D17, 5D23, and 3B17) progenitor cells. The vast majority of neurons contain vGlut1+ punctae in their neurites (labeled by Tuj1). Right panels are enlarged images from the insets of left panels.Data information: Scale bars, 50 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31566294), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
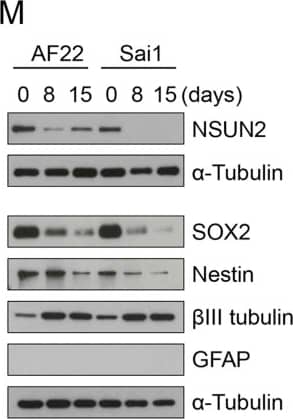
Expression of NSUN2 in the Human Developing Brain and NES Cells(A) DAPI-stained human embryo (6 weeks of gestation) marked for prosencephalon, mesencephalon, and rhombencephalon. Region in square is magnified in (B). Scale bar, 1 mm.(B) Prosencephalon labeled for NSUN2 and SOX1. Region in squares are magnified in (b′) and (b″). Arrows indicate NSUN2-positive cells. Scale bar, 100 μm.(C–F) Bright-field image (C) and immunofluorescence (D–F) of AF22 (upper panels) and Sai1 (lower panels) cells labeled for Nestin (D), SOX2 (E), and betaIII-tubulin (F). Scale bar, 50 μm.(G and H) NES cells co-labeled for NSUN2 and Nestin (NES) (G) or SOX1 (H).(I) Differentiation protocol.(J–L) Differentiated AF22 and Sai1 cells (day 15) labeled for Nestin (NES; J), SOX2 (K), and betaIII-tubulin (L). Scale bars: 50 μm.(M) Western blot for NSUN2, betaIII-tubulin (TUBB3), GFAP, SOX2, and Nestin during differentiation (days). alpha-Tubulin served as loading control.Nuclei are counterstained with DAPI (A, B, D–F, J–L). Image collected and cropped by CiteAb from the following publication (https://linkinghub.elsevier.com/retrieve/pii/S2213671116302764), licensed under a CC-BY license. Not internally tested by R&D Systems.
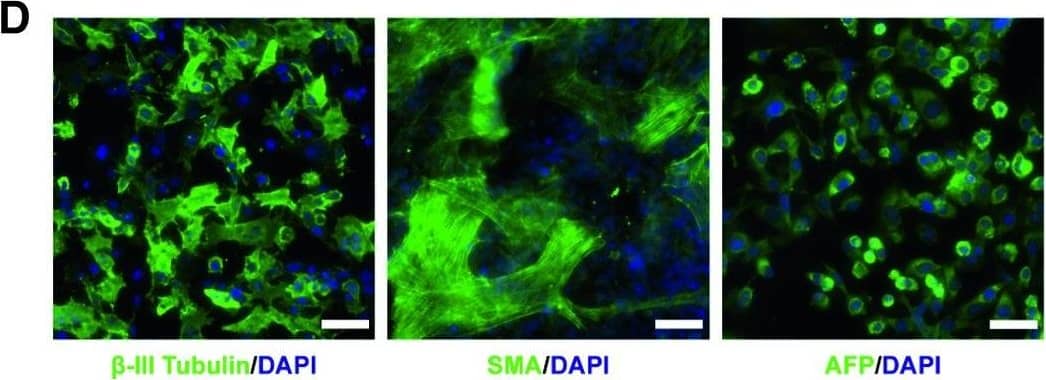
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Functional glycosylation of alpha‐dystroglycan and characterization of dystroglycanopathy patient‐specific iPSCsCurrent model of the core M3 functional glycan structure on alpha‐dystroglycan and enzymes involved in its synthesis. ECM ligands, such as laminins, bind to the Xyl‐GlucA disaccharide repeats (IIH6 epitope). Man, mannose; GlcNAc, N‐acetylglucosamine; GalNAc, N‐acetylgalactosamine; Rbo5P, ribitol‐5‐phosphate; Xyl, xylose; GlcA, glucuronic acid.Representative images of immunostaining demonstrate that FKRP‐iPSCs express specific pluripotency‐associated markers, including NANOG, OCT4, Tra‐1‐60, and SSEA4.FKRP‐iPSCs have a normal karyotype.In vitro differentiation of FKRP‐iPSCs to cells representing ectoderm ( beta‐III Tubulin, Tuj1), mesoderm (SMA, smooth muscle actin), and endoderm (AFP, alpha‐fetoprotein).Data information: Scale bars, 50 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31566294), licensed under a CC-BY license. Not internally tested by R&D Systems.
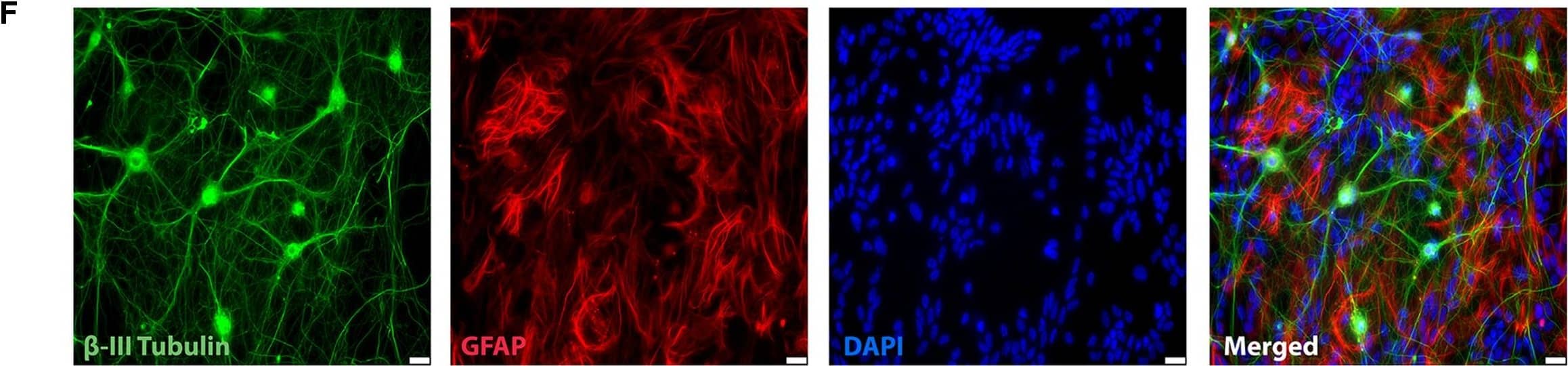
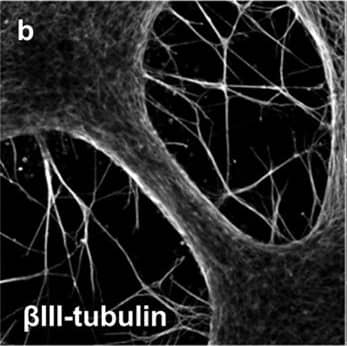
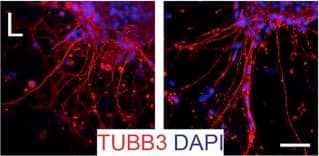
Detection of Mouse beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Representative immunocytochemistry demonstrating the expression of neuronal, synaptic, glutamatergic, GABAergic, and glial markers in cultures 21 days after plating. Neuronal cultures were immunostained against beta-III Tubulin (A–F); SNAP-25 (A); SV2 isoform A–C (A,B); polysialo gangliosides GD1a and GT1b/2b (C) Syn1 (D,E); glutamatergic neurons (VGLUT2, D); GABAergic neurons (GAD1, E) and glial cells (GFAP, F). Shown also are DAPI nuclear staining and the merged images. Scale bar is 10 μm (A–E), respectively, 25 μm for the bottom panel (F). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28280466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Representative immunocytochemistry demonstrating the expression of neuronal, synaptic, glutamatergic, GABAergic, and glial markers in cultures 21 days after plating. Neuronal cultures were immunostained against beta-III Tubulin (A–F); SNAP-25 (A); SV2 isoform A–C (A,B); polysialo gangliosides GD1a and GT1b/2b (C) Syn1 (D,E); glutamatergic neurons (VGLUT2, D); GABAergic neurons (GAD1, E) and glial cells (GFAP, F). Shown also are DAPI nuclear staining and the merged images. Scale bar is 10 μm (A–E), respectively, 25 μm for the bottom panel (F). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28280466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-III Tubulin by Western Blot
Expression of NSUN2 in the Human Developing Brain and NES Cells(A) DAPI-stained human embryo (6 weeks of gestation) marked for prosencephalon, mesencephalon, and rhombencephalon. Region in square is magnified in (B). Scale bar, 1 mm.(B) Prosencephalon labeled for NSUN2 and SOX1. Region in squares are magnified in (b′) and (b″). Arrows indicate NSUN2-positive cells. Scale bar, 100 μm.(C–F) Bright-field image (C) and immunofluorescence (D–F) of AF22 (upper panels) and Sai1 (lower panels) cells labeled for Nestin (D), SOX2 (E), and betaIII-tubulin (F). Scale bar, 50 μm.(G and H) NES cells co-labeled for NSUN2 and Nestin (NES) (G) or SOX1 (H).(I) Differentiation protocol.(J–L) Differentiated AF22 and Sai1 cells (day 15) labeled for Nestin (NES; J), SOX2 (K), and betaIII-tubulin (L). Scale bars: 50 μm.(M) Western blot for NSUN2, betaIII-tubulin (TUBB3), GFAP, SOX2, and Nestin during differentiation (days). alpha-Tubulin served as loading control.Nuclei are counterstained with DAPI (A, B, D–F, J–L). Image collected and cropped by CiteAb from the following publication (https://linkinghub.elsevier.com/retrieve/pii/S2213671116302764), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Characterization of NSCs and cortical neurons derived from FKRP‐ and CRISPR/Cas9 corrected‐iPSCsA, BRepresentative images of NSCs derived from FKRP‐ and corrected‐iPSC lines expressing SOX1, SOX2, and nestin.C, DQuantification of percentage of SOX1+ (C) and SOX2+ (D) cells in culture. The efficiency of neural induction is more than 99% in FKRP‐ and corrected‐iPSC (5D17, 5D23, and 3B17) lines. Data are mean ± s.d. n = 4 technical replicates.E, FFKRP‐ and corrected‐NSC lines can be further differentiated to cortical neural progenitor cells, expressing PAX6, OTX2, and vimentin.G–IQuantification of percentage of PAX6+ (G) and OTX2+ (H) cells in culture. About 91‐98% of cells derived from FKRP, 5D17, 5D23, and 3B17 NSC lines express PAX6 (G). About 93‐96% of cells derived from FKRP, 5D17, 5D23, and 3B17 NSC lines express OTX2 (H). Of the OTX2+ population, about 60‐67% cells are also Ki67+ cycling progenitors (I). Data are mean ± s.d. n = 4 technical replicates.J, KGlutamatergic projection neurons derived from FKRP and corrected (5D17, 5D23, and 3B17) progenitor cells. The vast majority of neurons contain vGlut1+ punctae in their neurites (labeled by Tuj1). Right panels are enlarged images from the insets of left panels.Data information: Scale bars, 50 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31566294), licensed under a CC-BY license. Not internally tested by R&D Systems.
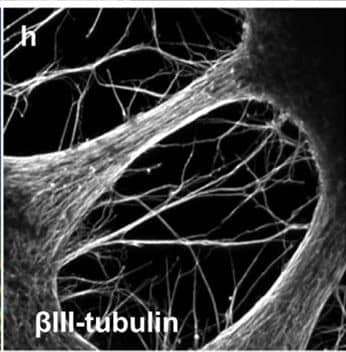
Detection of Mouse beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Representative immunocytochemistry demonstrating the expression of neuronal, synaptic, glutamatergic, GABAergic, and glial markers in cultures 21 days after plating. Neuronal cultures were immunostained against beta-III Tubulin (A–F); SNAP-25 (A); SV2 isoform A–C (A,B); polysialo gangliosides GD1a and GT1b/2b (C) Syn1 (D,E); glutamatergic neurons (VGLUT2, D); GABAergic neurons (GAD1, E) and glial cells (GFAP, F). Shown also are DAPI nuclear staining and the merged images. Scale bar is 10 μm (A–E), respectively, 25 μm for the bottom panel (F). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28280466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Representative immunocytochemistry demonstrating the expression of neuronal, synaptic, glutamatergic, GABAergic, and glial markers in cultures 21 days after plating. Neuronal cultures were immunostained against beta-III Tubulin (A–F); SNAP-25 (A); SV2 isoform A–C (A,B); polysialo gangliosides GD1a and GT1b/2b (C) Syn1 (D,E); glutamatergic neurons (VGLUT2, D); GABAergic neurons (GAD1, E) and glial cells (GFAP, F). Shown also are DAPI nuclear staining and the merged images. Scale bar is 10 μm (A–E), respectively, 25 μm for the bottom panel (F). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28280466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Neuronal markers expressed by iPSC-NSCs after differentiation and maturation on PEG hydrogels.(a) betaIII-tubulin (red), GAD 65/67 (green), and DAPI (nuclei, blue). Single channel grayscale images from (a) are shown for (b) betaIII-tubulin and (c) GAD 65/67. (d) VGLUT2 (red), MAP2 (green), and DAPI (nuclei, blue). Single channel grayscale images from (d) are shown for (e) VGLUT2 and (f) MAP2. (g) betaIII-tubulin (red), GABA (green), and DAPI (nuclei, blue). Single channel grayscale images from (g) are shown for (h) betaIII-tubulin and (i) GABA. (j) MNX1/HB9 (green) and DAPI (nuclei, blue). Single channel grayscale images from (j) are shown for (k) MNX1/HB9 and (l) DAPI. (m) betaIII-tubulin (red), Synapsophysin (green), and DAPI (nuclei, blue). Single channel grayscale images from (m) are shown for (n) betaIII-tubulin and (o) Synapsophysin. (p) Synapsin-1 (green) and DAPI (nuclei, blue). Single channel grayscale images from (p) are shown for (q) Synapsin-1 and (r) DAPI. Scale Bars: 200 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26411797), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Representative immunocytochemistry demonstrating the expression of neuronal, synaptic, glutamatergic, GABAergic, and glial markers in cultures 21 days after plating. Neuronal cultures were immunostained against beta-III Tubulin (A–F); SNAP-25 (A); SV2 isoform A–C (A,B); polysialo gangliosides GD1a and GT1b/2b (C) Syn1 (D,E); glutamatergic neurons (VGLUT2, D); GABAergic neurons (GAD1, E) and glial cells (GFAP, F). Shown also are DAPI nuclear staining and the merged images. Scale bar is 10 μm (A–E), respectively, 25 μm for the bottom panel (F). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28280466), licensed under a CC-BY license. Not internally tested by R&D Systems.
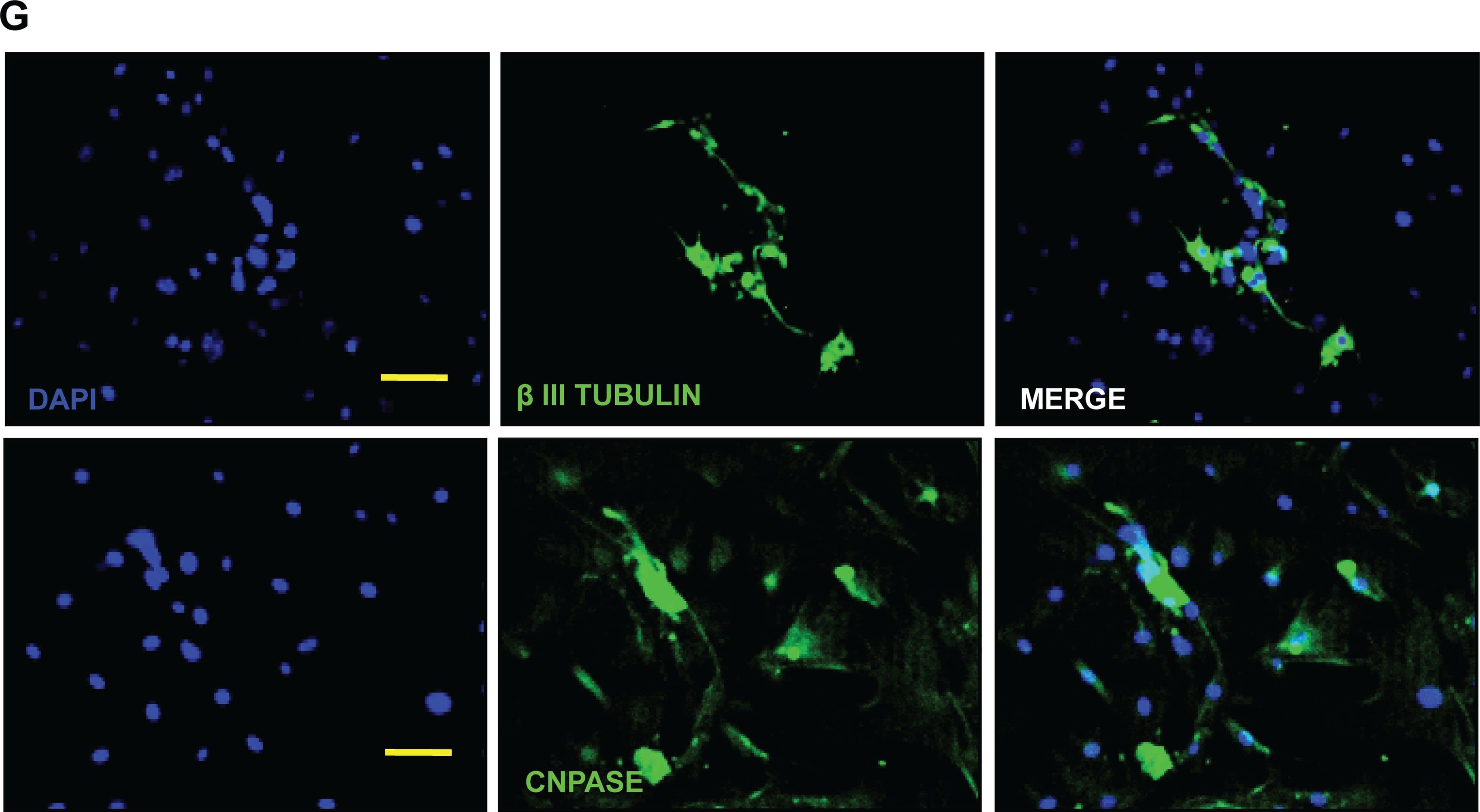
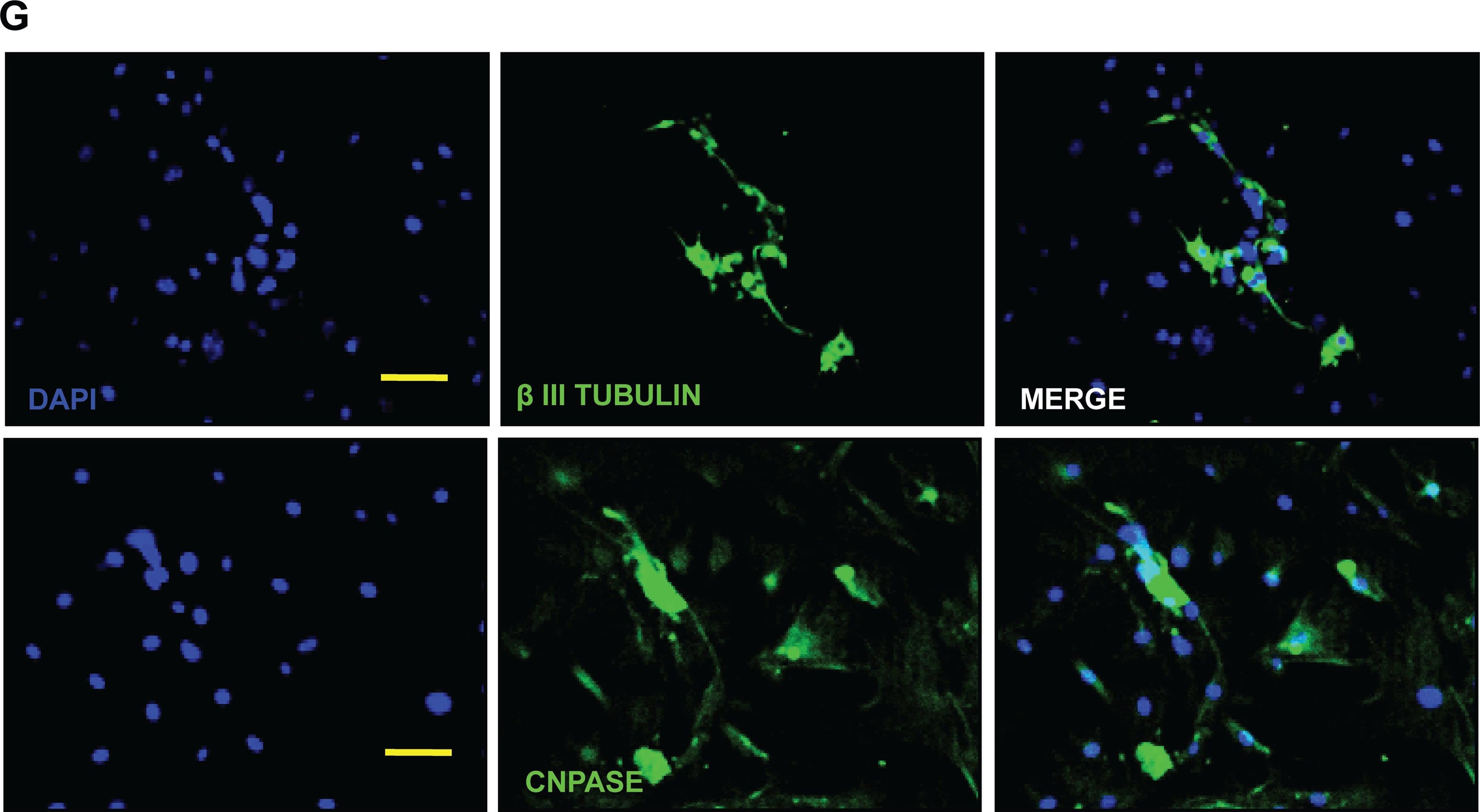
Detection of Mouse beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Differentiation of murine skin stem cell precursors (SKP).The differentiation of SKPs was induced through the dissociation of cell aggregates into single cells followed by serum exposure and, as may be seen in the micrographs after 7 days (A), 14 days (B) and 21 days (C), these cells presented several elongated extensions. The mRNA levels of astrocyte markers (D) GFAP; oligodendrocytes (E) CNPase; and neurons (F) betaIII tubulin were measured by qRT-PCR and validated at the protein level (G) by immunofluorescence microscopy, Bar = 50μm. The results are presented as the mean ± SD of values obtained in three independent experiments performed in triplicates. Statistical analyzes were performed using ANOVA analysis of variance followed by Tukey test to post-Kramer. All groups were measured versus the undifferentiated control in the shortest time of differentiation, ns = not significant; * p ≤ 0.05; ** p≤0.001. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0140143), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Neuronal markers expressed by iPSC-NSCs after differentiation and maturation on PEG hydrogels.(a) betaIII-tubulin (red), GAD 65/67 (green), and DAPI (nuclei, blue). Single channel grayscale images from (a) are shown for (b) betaIII-tubulin and (c) GAD 65/67. (d) VGLUT2 (red), MAP2 (green), and DAPI (nuclei, blue). Single channel grayscale images from (d) are shown for (e) VGLUT2 and (f) MAP2. (g) betaIII-tubulin (red), GABA (green), and DAPI (nuclei, blue). Single channel grayscale images from (g) are shown for (h) betaIII-tubulin and (i) GABA. (j) MNX1/HB9 (green) and DAPI (nuclei, blue). Single channel grayscale images from (j) are shown for (k) MNX1/HB9 and (l) DAPI. (m) betaIII-tubulin (red), Synapsophysin (green), and DAPI (nuclei, blue). Single channel grayscale images from (m) are shown for (n) betaIII-tubulin and (o) Synapsophysin. (p) Synapsin-1 (green) and DAPI (nuclei, blue). Single channel grayscale images from (p) are shown for (q) Synapsin-1 and (r) DAPI. Scale Bars: 200 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26411797), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Neuronal markers expressed by iPSC-NSCs after differentiation and maturation on PEG hydrogels.(a) betaIII-tubulin (red), GAD 65/67 (green), and DAPI (nuclei, blue). Single channel grayscale images from (a) are shown for (b) betaIII-tubulin and (c) GAD 65/67. (d) VGLUT2 (red), MAP2 (green), and DAPI (nuclei, blue). Single channel grayscale images from (d) are shown for (e) VGLUT2 and (f) MAP2. (g) betaIII-tubulin (red), GABA (green), and DAPI (nuclei, blue). Single channel grayscale images from (g) are shown for (h) betaIII-tubulin and (i) GABA. (j) MNX1/HB9 (green) and DAPI (nuclei, blue). Single channel grayscale images from (j) are shown for (k) MNX1/HB9 and (l) DAPI. (m) betaIII-tubulin (red), Synapsophysin (green), and DAPI (nuclei, blue). Single channel grayscale images from (m) are shown for (n) betaIII-tubulin and (o) Synapsophysin. (p) Synapsin-1 (green) and DAPI (nuclei, blue). Single channel grayscale images from (p) are shown for (q) Synapsin-1 and (r) DAPI. Scale Bars: 200 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26411797), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Expression of NSUN2 in the Human Developing Brain and NES Cells(A) DAPI-stained human embryo (6 weeks of gestation) marked for prosencephalon, mesencephalon, and rhombencephalon. Region in square is magnified in (B). Scale bar, 1 mm.(B) Prosencephalon labeled for NSUN2 and SOX1. Region in squares are magnified in (b′) and (b″). Arrows indicate NSUN2-positive cells. Scale bar, 100 μm.(C–F) Bright-field image (C) and immunofluorescence (D–F) of AF22 (upper panels) and Sai1 (lower panels) cells labeled for Nestin (D), SOX2 (E), and betaIII-tubulin (F). Scale bar, 50 μm.(G and H) NES cells co-labeled for NSUN2 and Nestin (NES) (G) or SOX1 (H).(I) Differentiation protocol.(J–L) Differentiated AF22 and Sai1 cells (day 15) labeled for Nestin (NES; J), SOX2 (K), and betaIII-tubulin (L). Scale bars: 50 μm.(M) Western blot for NSUN2, betaIII-tubulin (TUBB3), GFAP, SOX2, and Nestin during differentiation (days). alpha-Tubulin served as loading control.Nuclei are counterstained with DAPI (A, B, D–F, J–L). Image collected and cropped by CiteAb from the following publication (https://linkinghub.elsevier.com/retrieve/pii/S2213671116302764), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-III Tubulin by Immunocytochemistry/Immunofluorescence
Neuronal markers expressed by iPSC-NSCs after differentiation and maturation on PEG hydrogels.(a) betaIII-tubulin (red), GAD 65/67 (green), and DAPI (nuclei, blue). Single channel grayscale images from (a) are shown for (b) betaIII-tubulin and (c) GAD 65/67. (d) VGLUT2 (red), MAP2 (green), and DAPI (nuclei, blue). Single channel grayscale images from (d) are shown for (e) VGLUT2 and (f) MAP2. (g) betaIII-tubulin (red), GABA (green), and DAPI (nuclei, blue). Single channel grayscale images from (g) are shown for (h) betaIII-tubulin and (i) GABA. (j) MNX1/HB9 (green) and DAPI (nuclei, blue). Single channel grayscale images from (j) are shown for (k) MNX1/HB9 and (l) DAPI. (m) betaIII-tubulin (red), Synapsophysin (green), and DAPI (nuclei, blue). Single channel grayscale images from (m) are shown for (n) betaIII-tubulin and (o) Synapsophysin. (p) Synapsin-1 (green) and DAPI (nuclei, blue). Single channel grayscale images from (p) are shown for (q) Synapsin-1 and (r) DAPI. Scale Bars: 200 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26411797), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Neuron-specific beta-III Tubulin Antibody by Immunocytochemistry/ Immunofluorescence
Differentiation of murine skin stem cell precursors (SKP).The differentiation of SKPs was induced through the dissociation of cell aggregates into single cells followed by serum exposure and, as may be seen in the micrographs after 7 days (A), 14 days (B) and 21 days (C), these cells presented several elongated extensions. The mRNA levels of astrocyte markers (D) GFAP; oligodendrocytes (E) CNPase; and neurons (F) betaIII tubulin were measured by qRT-PCR and validated at the protein level (G) by immunofluorescence microscopy, Bar = 50μm. The results are presented as the mean ± SD of values obtained in three independent experiments performed in triplicates. Statistical analyzes were performed using ANOVA analysis of variance followed by Tukey test to post-Kramer. All groups were measured versus the undifferentiated control in the shortest time of differentiation, ns = not significant; * p ≤ 0.05; ** p≤0.001. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26462205), licensed under a CC-BY license. Not internally tested by R&D Systems.
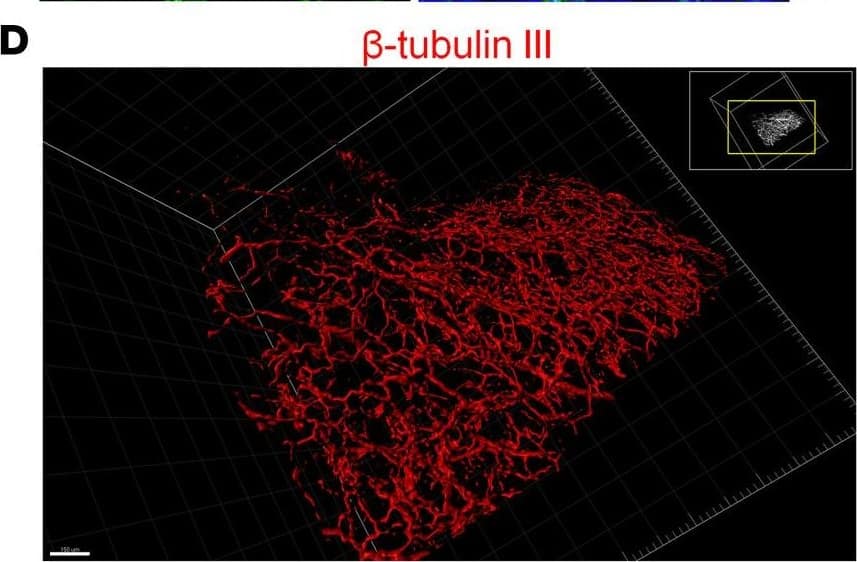
Detection of Mouse Neuron-specific beta-III Tubulin Antibody by Immunohistochemistry
CTCL is associated with nerve innervations in mouse lymphoma.(A) Double immunostaining for CGRP and beta–tubulin III in the back skin with lymphoma from a CTCL mouse at day 20. Dotted lines show the epidermis. Scale bar: 25 μm. (B) Immunostaining for NF200 in the tumor from a CTCL mouse at day 20. Dotted lines show the epidermis. Scale bar: 25 μm. (C) 3D reconstruction of innervated nerves in a beta–tubulin III–labeled tumor from a CTCL mouse at day 20. Scale bar: 300 μm. (D) High-magnification image of the boxed area in C. Scale bar: 150 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/36520531), licensed under a CC-BY license. Not internally tested by R&D Systems.
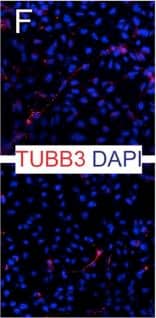
Detection of Human Neuron-specific beta-III Tubulin Antibody by Immunocytochemistry/ Immunofluorescence
Expression of NSUN2 in the Human Developing Brain and NES Cells(A) DAPI-stained human embryo (6 weeks of gestation) marked for prosencephalon, mesencephalon, and rhombencephalon. Region in square is magnified in (B). Scale bar, 1 mm.(B) Prosencephalon labeled for NSUN2 and SOX1. Region in squares are magnified in (b′) and (b″). Arrows indicate NSUN2-positive cells. Scale bar, 100 μm.(C–F) Bright-field image (C) and immunofluorescence (D–F) of AF22 (upper panels) and Sai1 (lower panels) cells labeled for Nestin (D), SOX2 (E), and betaIII-tubulin (F). Scale bar, 50 μm.(G and H) NES cells co-labeled for NSUN2 and Nestin (NES) (G) or SOX1 (H).(I) Differentiation protocol.(J–L) Differentiated AF22 and Sai1 cells (day 15) labeled for Nestin (NES; J), SOX2 (K), and betaIII-tubulin (L). Scale bars: 50 μm.(M) Western blot for NSUN2, betaIII-tubulin (TUBB3), GFAP, SOX2, and Nestin during differentiation (days). alpha-Tubulin served as loading control.Nuclei are counterstained with DAPI (A, B, D–F, J–L). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28041877), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human Neuron-specific beta-III Tubulin Antibody by Immunocytochemistry/ Immunofluorescence
I alphaI maintains pluripotency and differentiation capacity in long-term culture.Immunofluorescence staining of H207 hES cell line for (a) stem cell markers Oct4 (green), Sox2 (green), Nanog (red) and SSEA-4 (green) after 16 passages in E8:I alphaI, and for the three germ layers after 4 weeks of differentiation through embryoid body (EB) formation: mesoderm with alpha smooth muscle actin (SMA, green), ectoderm with Nestin (red) and beta-III-tubulin (green) and endoderm with alpha-fetoprotein (AFP, green) and PDGF-receptor (red), and (b) after endoderm directed differentiation for endoderm markers Sox7 (red), Sox17 (green) and HNF3 beta (green) and stem cell markers Oct4 (green) and Nanog (red), as well as negative control with secondary antibodies. All samples were co-stained with DAPI (Blue) for nuclei detection. Scale bars show 100 μm, white boxes show close-up images. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27405751), licensed under a CC-BY license. Not internally tested by R&D Systems.
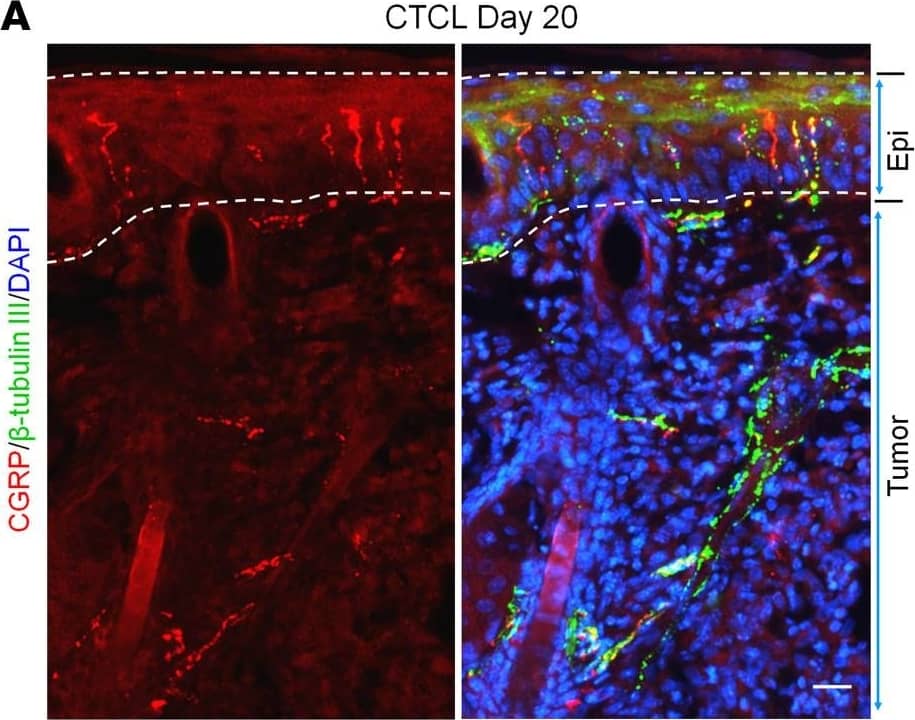
Detection of Mouse Neuron-specific beta-III Tubulin Antibody by Immunohistochemistry
CTCL is associated with nerve innervations in mouse lymphoma.(A) Double immunostaining for CGRP and beta–tubulin III in the back skin with lymphoma from a CTCL mouse at day 20. Dotted lines show the epidermis. Scale bar: 25 μm. (B) Immunostaining for NF200 in the tumor from a CTCL mouse at day 20. Dotted lines show the epidermis. Scale bar: 25 μm. (C) 3D reconstruction of innervated nerves in a beta–tubulin III–labeled tumor from a CTCL mouse at day 20. Scale bar: 300 μm. (D) High-magnification image of the boxed area in C. Scale bar: 150 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/36520531), licensed under a CC-BY license. Not internally tested by R&D Systems.