Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein, CF Best Seller
R&D Systems, part of Bio-Techne | Catalog # 3718-FB


Now offering a heat stable form of Recombinant Human FGF basic (Catalog # BT-FGFBHS) that retains activity at incubator temperatures and adds flexibility to your media change intervals.
Key Product Details
Product Specifications
Source
Ala144-Ser288
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
Activity
The ED50 for this effect is 0.100-0.600 ng/mL.
Reviewed Applications
Read 2 reviews rated 4.5 using 3718-FB in the following applications:
Scientific Data Images for Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein, CF
Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein Bioactivity
Measured in a cell proliferation assay using NR6R‑3T3 mouse fibroblast cells. Raines, E.W.et al. (1985) Methods Enzymol.109:749. The ED50 for this effect is 0.100-0.600 ng/mL.iPSC-derived Cerebral Organoid Culture using Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein.
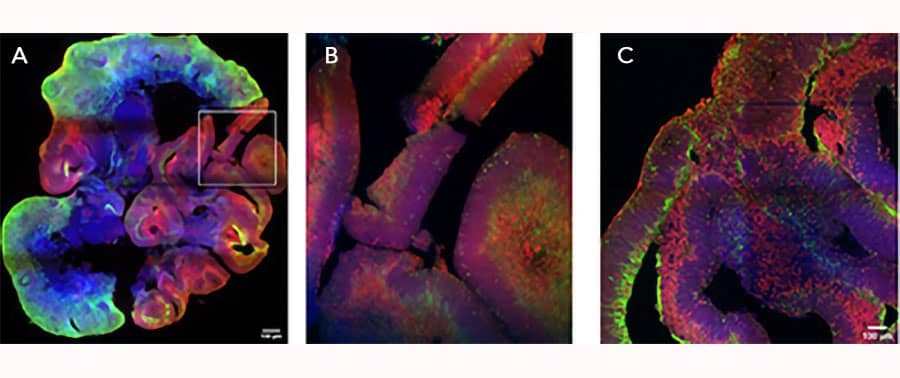
iPSC-derived cerebral organoids (day 45) were cultured using Cultrex UltiMatrix RGF Basement Membrane Extract (BME001-05) and brain organoid culture medium, which includes Recombinant Human FGF-basic (Catalog # 3718-FB) and Recombinant Human Noggin (6057-NG), along with the other reagents listed in the brain organoid culture recipe. Cerebral organoids were stained for Syto6 (blue), Pax6 (red), and Vimentin (green). (A) Image taken at 4x magnification. (B) An enlarged view of the area shown within the white box in part A of the figure. (C) Image taken at 15x magnification. Images courtesy of LifeCanvas Technologies.iPSC-derived Cerebral Organoid Culture using Recombinant Human FGFbasic/FGF2/bFGF (145 aa) Protein.
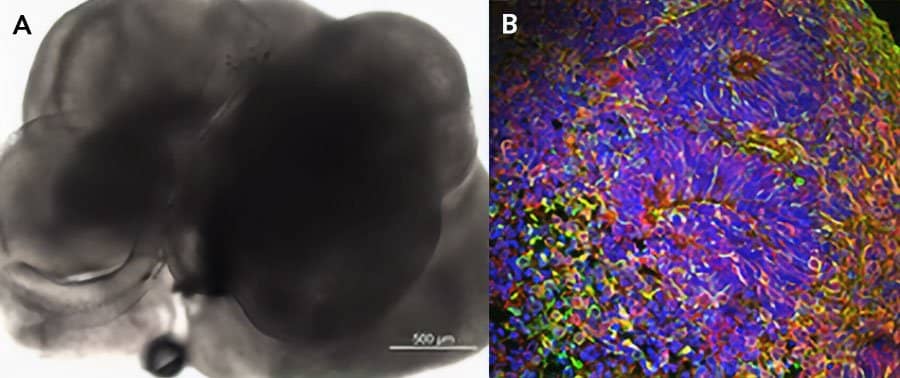
iPSC-derived cerebral organoids (day 45) were cultured using Cultrex UltiMatrix RGF Basement Membrane Extract (BME001-05) and brain organoid culture medium, which includes Recombinant Human FGF-basic (Catalog # 3718-FG) and Recombinant Human Noggin (6057-NG), along with the other reagents listed in the brain organoid culture recipe. (A) Representative brightfield image of day 30 iPSC-derived cerebral organoids. (B) Cerebral organoids stained for beta III-tubulin (green) and Prox1 (red), and counterstained with DAPI (blue).Formulation, Preparation and Storage
3718-FB
| Formulation | Lyophilized from a 0.2 μm filtered solution in Tris-HCl and NaCl. |
| Reconstitution |
Reconstitute at 100 μg/mL in sterile PBS.
|
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: FGF basic/FGF2/bFGF
FGF basic (also known as FGF-2 and HBGF-2) is an 18-34 kDa, heparin-binding member of the FGF superfamily of molecules (1-3). Superfamily members are characterized by the presence of a centrally placed beta-trefoil structure. FGF acidic (FGF-1) and FGF basic (FGF-2) were the first two identified FGFs, and the designations acidic and basic refer to their relative isoelectric points. Human FGF basic is 288 amino acids (aa) in length. There are multiple start sites, four of which utilize atypical CUG codons, and one that initiates at an AUG start site (4-6). The four CUG start sites generate high molecular weight (HMW) FGF basic. There is a 34 kDa, 288 aa form, a 24 kDa, 210 aa form, a 22.5 kDa, 201 aa form, and a 22 kDa, 196 aa form. All are retained intracellularly, undergo extensive methylation, and possess one or more nuclear localization signals (NLS) (7-9). The AUG initiating form is 18 kDa and 155 aa in length. There is no signal sequence (ss). It is, however, secreted directly through the plasma membrane via a mechanism that appears to be dependent upon tertiary structure (10). In place of a ss, there is purportedly a 9 aa N-terminal prosegment that precedes a 146 aa mature segment (11). Early isolations of 18 kDa bovine FGF basic yielded 146 aa molecules, an effect attributed to the presence of acid proteases (12). The molecule contains a heparin-binding site (aa residues 128-144), and undergoes phosphorylation at Ser117 (13). There is also an ill-defined C-terminal NLS that may be more “functional” (or 3-dimensional) than structural (7). Human 146 aa FGF basic is 97% aa identical to mouse FGF basic (14).
References
- Sorenson, V. et al. (2006) BioEssays 28:504.
- Kardami, E. et al. (2004) Cardiovasc. Res. 63:458.
- Nugent, M.A. and R.V. Lozzo (2000) Int. J. Biochem. Cell Biol. 32:115.
- Abraham, J.A. et al. (1986) EMBO J. 5:2523.
- Prats, H. et al. (1989) Proc. Natl. Acad. Sci. USA 86:1836.
- Arnaud, E. et al. (1999) Mol. Cell. Biol. 19:505.
- Foletti, A. et al. (2003) Cell. Mol. Life Sci. 60:2254.
- Arese, M. et al. (1999) Mol. Biol. Cell 10:1429.
- Pintucci, G. et al. (1996) Mol. Biol. Cell 7:1249.
- Nickel, W. (2005) Traffic 6:607.
- SwissProt # P09038.
- Klagsbrun, M. et al. (1987) Proc. Natl. Acad. Sci. USA 84:1839.
- Bailly, K. et al. (2000) FASEB J. 14:333.
- Hebert, J.M. et al. (1990) Dev. Biol. 138:454.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional FGF basic/FGF2/bFGF Products
Product Documents for Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein, CF
Product Specific Notices for Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein, CF
For research use only