Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein Best Seller
R&D Systems, part of Bio-Techne | Catalog # 234-FSE


Now offering a heat stable form of Recombinant Human FGF basic (Catalog # BT-FGFBHS) that retains activity at incubator temperatures and adds flexibility to your media change intervals.
Key Product Details
Product Specifications
Source
Gly132-Ser288
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
Activity
The ED50 for this effect is 0.5-2.5 ng/mL.
Reviewed Applications
Read 2 reviews rated 5 using 234-FSE in the following applications:
Scientific Data Images for Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein
Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein Bioactivity
Recombinant Human FGF basic/FGF2/bFGF (157 aa) (Catalog # 234-FSE) stimulates cell proliferation of the NR6R‑3T3 mouse fibroblast cell line. The ED50 for this effect is 0.5-2.5 ng/mL.Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein SDS-PAGE
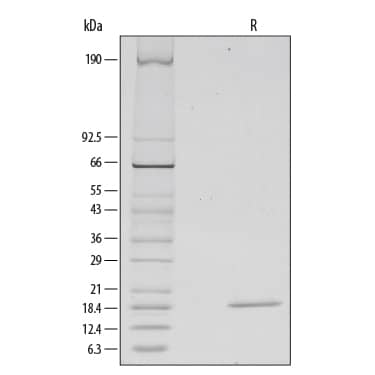
1 μg/lane of Recombinant Human FGF basic/FGF2/bFGF (157 aa) was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 19 kDa.Formulation, Preparation and Storage
Carrier Free
What does CF mean?CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
What formulation is right for me?In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
Carrier: 234-FSE
| Formulation | Lyophilized from a 0.2 μm filtered solution in Tris-HCl and NaCl with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Carrier Free: 234-FSE/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in Tris-HCl and NaCl. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: FGF basic/FGF2/bFGF
FGF basic is a member of the FGF family of at least 23 related mitogenic proteins which show 35-60% amino acid conservation. FGF acidic and basic, unlike the other members of the family, lack signal peptides and are apparently secreted by mechanisms other than the classical protein secretion pathway. FGF basic has been isolated from a number of sources, including neural tissue, pituitary, adrenal cortex, corpus luteum, and placenta. This factor contains four cysteine residues, but reduced FGF basic retains full biological activity, indicating that disulfide bonds are not required for this activity. A variety of forms of FGF basic are produced as a result of N-terminal extensions. These extensions affect localization of FGF basic in cellular compartments but do not affect biological activity. Binding of FGF to heparin or cell surface heparan sulfate proteoglycans is necessary for binding of FGF to high affinity FGF receptors. FGF acidic and basic appear to bind to the same high affinity receptors and show a similar range of biological activities. FGF basic stimulates the proliferation of all cells of mesodermal origin and many cells of neuroectodermal, ectodermal, and endodermal origin. FGF basic induces neuron differentiation, survival, and regeneration. FGF basic also modulates embryonic development and differentiation. These observed in vitro functions of FGF basic suggest FGF basic may play a role in vivo in the modulation of such normal processes as angiogenesis, wound healing and tissue repair, embryonic development and differentiation, and neuronal function and neural degeneration. Additionally, FGF basic may participate in the production of a variety of pathological conditions resulting from excessive cell proliferation and excessive angiogenesis.
References
- Coulier, F. et al. 1997, J. Mol. Evol. 44:43.
- Chen, C.H. et al. 2004, Curr. Vasc. Pharmacol. 2:33.
- Mohammadi, M. et al. 2005, Curr. Opin. Struct. Biol. 15:506.
- Fernig, D. et al. 1994, Prog. Growth Factor Res. 5:353.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional FGF basic/FGF2/bFGF Products
Product Documents for Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein
Product Specific Notices for Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein
For research use only