Advanced Western Blotting Solutions for Vaccine Development
Widely used to improve reproducibility, quantitation, throughput and time to market, Simple Western™ can help you develop and characterize your vaccine candidates. With the ability to separate proteins by Size or Charge, Simple Western offers unparalleled speed and insight leveraged throughout vaccine development workflows. Simple Western can be used to measure protein in crude cell lysates in upstream samples as well as in samples further downstream to detect impurities and assess final product release, stability, and characterization.

Simple Western Advantages in Vaccine Development
By offering automation, reproducibility, sensitivity, and quick time to results, Simple Western has proven to be an invaluable tool across the entire vaccine development pipeline.
- Quantitation. Truly quantitative immunoassays using conventional Western blot antibodies and built-in total protein normalization.
- Reproducibility. Eliminate the variation of traditional Western blots, with intra-assay CV’s of <15%.
- Sensitivity. Stellar™ NIR/IR Modules for Jess™ with industry-leading sensitivity for fluorescence Western-blotting workflows.
- Throughput. Process up to 96 samples per automated overnight run. Screen vaccine candidates with triplicate data points. Get even more data points per sample with RePlex™ and lower the cost per data point.
- Method transferability. Using Compass for Simple Western, all data and records are digital, allowing for easy analysis, transfer and archiving between sites or from sponsors to CROs.
- Compliance. 21 CFR Part 11 compliant, ready for your organization’s regulatory needs.

The Vaccine Development Workflow
Simple Western in Each Step
Learn how Simple Western was used to characterize the Zaire Ebola vaccine development in each step of the process and accelerate development of a critically needed vaccine amid a global health crisis.
Learn how scientists from Merck and Medgene Labs use Simple Western to assess proteins used in vaccine development for critical quality attributes such as identity and purity, that support the entire vaccine development process, from discovery to QC and lot release.
Identify and Screen Vaccine Candidates
Rapidly Screen Samples and Antibodies
“Simple Western allowed me to screen quickly lots of samples with different antibodies while eliminating the manual washing and incubation steps.”
- Dr. Matteo Ferrari, Postdoc, DIOSynVax, Department of Veterinary Medicine, Cambridge, United Kingdom

Survey the Humoral Response
Rich Multi-Analyte Characterization
Simple Western assays can help measure serum antibody levels to confirm immune responses against an antigen and to test for antibody production in response to vaccination. In this application note, we used Simple Western to detect autoantibodies in lupus patient serum as a model system to generate proof-of-concept data for the assay.


Compared to a single antigen assay, Simple Western serology assays can be used to detect serum antibodies against multiple different antigens simultaneously to give a more complete view into the humoral response. The SARS-CoV-2 Multi-Antigen Serology Module for Simple Western can be utilized to detect and quantify the humoral response to five key SARS-CoV-2 antigens in serum or plasma simultaneously in a single capillary while also providing molecular weight information of each antigen, all without compromising sensitivity.
Measure Vaccine Efficacy
Charge- and Size-Based Separation
Here, the Charge-based separation of Simple Western was utilized to quantitatively to assess the charge heterogeneities of gH/gL and pentameric complex, purity of gH/gL/pUL128–131 pentameric complex, and confirm the immunoassay specificity. The published results highlight the importance of the gH/gL/pUL128-131 PC in HCMV vaccine design and emphasize the necessity to monitor the integrity of the PC during vaccine manufacturing process.

Monitor Vaccine Quality
Contaminant Detection
Simple Western assays have already been optimized for characterization of several different critical quality attributes including detecting process-related contaminants like host cell proteins, Protein A, GFP, and BSA, making it easy for you to get Simple Western integrated into your cell therapy workflow. In this application note, learn more about how Simple Western platforms detect contaminants in denatured and reduced samples, with the sensitivity needed to confirm if their concentration falls below regulatory guidelines.
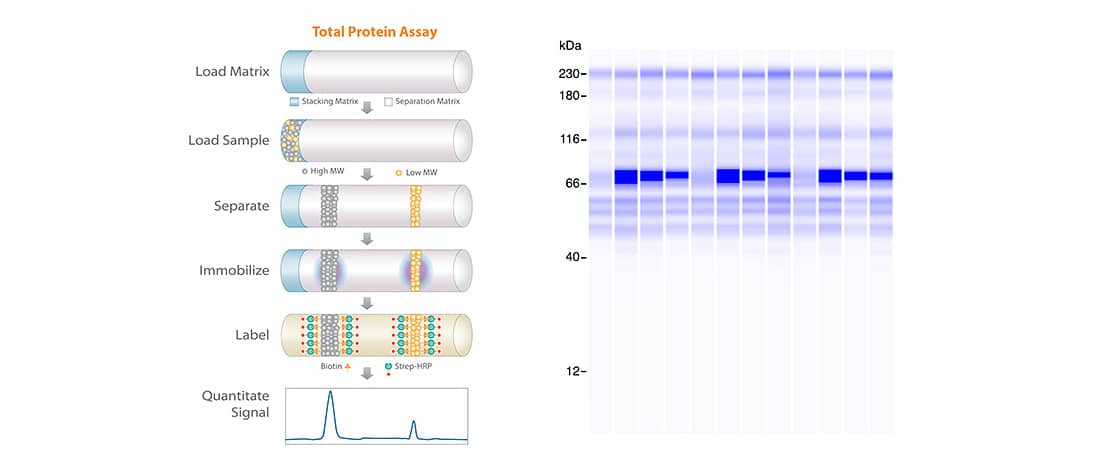
Total Protein - More Sensitive than SYPRO Ruby
Purity of the final vaccine product is often confirmed using detection methods like SYPRO Ruby or Coomassie staining methods which reportedly require lower nanogram levels for reliable detection. The precision, sensitivity and flexibility of Simple Western surpasses traditional approaches for detecting process-related contaminants. The 5X total protein labeling reagent in conjunction with RePlex to achieve ultrasensitive total protein detection enables researchers to detect low abundance impurities, get the most data out of their precious samples, and normalize their protein expression data with confidence.
Monitor Vaccine Stability of Polysaccharide-Protein Conjugate Vaccines
Capsular polysaccharides derived from the surface of pathogenic bacteria can be used as antigen targets for vaccines. Scientists at Merck & Co., Inc., rely on Simple Western to monitor the stability of polysaccharide-protein conjugate vaccines, with the added advantage of being able to detect degradation products at very low concentration and under full automation, can reduce potential analysis error and analyst benchwork while having the benefits of speed and higher throughput with 96 capillaries.
Increase analytical capacity. A higher volume of information in a relatively short time.
Increase product coverage for a wide spectrum of vaccine product development. Discovery, preclinical, clinical, and commercialization in both the biopharmaceutical and vaccine industries.













